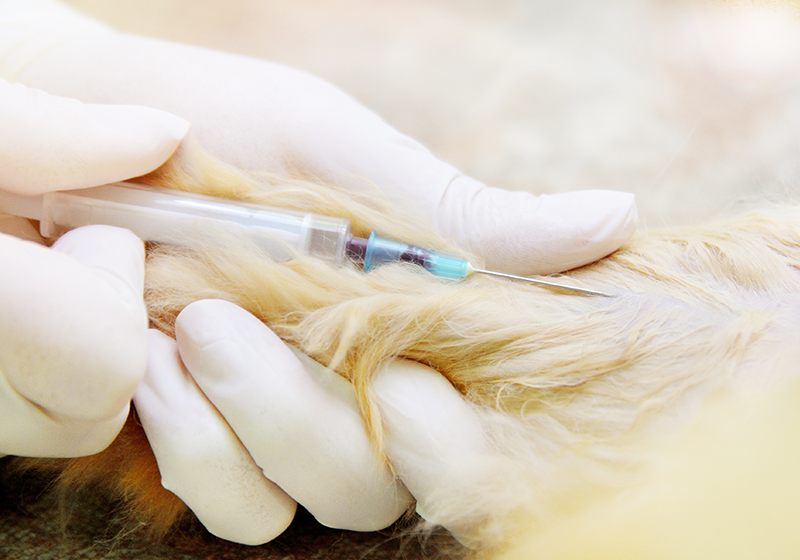
To develop veterinary drugs, it is necessary to understand the process from the discovery of active ingredients to product approval. Depending on the characteristics of the veterinary drug to be developed, the data required for product approval and the approach will also vary.
HLB bioStep is an institution designated by the Animal and Plant Quarantine Agency to conduct non-clinical and clinical trials for veterinary drugs.
We provide a service, based on veterinarians, that consults on the procedures and data review necessary for product approval, the development of clinical trials based on non-clinical efficacy tests, and clinical trial permissions, and performs clinical trials.
HLB bioStep is an institution designated by the Animal and Plant Quarantine Agency to conduct clinical/non-clinical trials for veterinary drugs.
-

Consulting for the development of veterinary drugs
-


Non-clinical trial services for veterinary drugs
-


Clinical trial services for veterinary drugs